I. Introduction: From “Risk Warning” to “Safety Revolution”
In the “last mile” of drug therapy, a seemingly insignificant component—the Low fragmentation medical stopper—plays the ultimate role of guarding the lifeline. However, this line of defense has long been plagued by two “silent killers”: microscopic fragments generated during Needle puncture resistant stopper performance failures, and unforeseen chemical reactions between the stopper and the drug.
This is not alarmism. Invisible rubber particles entering the human body with the solution can potentially cause capillary embolism, granulomas, or unpredictable immune reactions, representing a latent risk for pharmaceutical companies regarding quality traceability and product recalls. More insidiously, certain highly active drug molecules may adsorb to or react with impurities or leachables from the stopper, leading to diminished efficacy, altered impurity profiles, and directly challenging the stability and safety of the drug product, even jeopardizing the outcomes of costly clinical trials. The industry urgently requires advanced solutions from leading Rubber Functional filler Manufacturers to address these foundational challenges.
Faced with these industry ailments, traditional material solutions often fall short, addressing one issue while neglecting another. Today, a quiet revolution stemming from materials science is underway. This article delves into how the application of GreenThinking® RS series high-purity Reinforced silica medical stoppers—specifically RS915 and RS925—can fundamentally build a more robust, pure, and reliable pharmaceutical packaging system, armoring your core products with a tailored “nano-protective suit.”

II. Core Argument: Building a “Three-Dimensional Safety Fortress” for Pharmaceutical Packaging
Part 1: In-Depth Analysis – Why the Stopper is a “Critical Risk Point” in the Modern Drug Supply Chain
- Fragmentation Risk: From Physical Contamination to Biocompatibility Crisis
- Fragmentation is more than just “dust.” Each puncture is the ultimate test of the material’s toughness and integrity. The generated rubber particles not only risk blocking infusion pathways but, more critically, their unknown biocompatibility poses a Damocles’ sword over pharmaceutical manufacturers. Under increasingly stringent regulations, superior Abrasion resistant rubber stopper filler performance and tear strength have become the entry threshold for medical stoppers. The goal is to Reduce fragmentation in vial stoppers at the source.
- Chemical Reaction Hazards: The Silent Erosion of Efficacy and Stability
- Especially for sensitive drugs like biologics, monoclonal antibodies, vaccines, and highly potent APIs, the chemical inertness of the stopper is paramount. Metal ion catalysis (e.g., from Fe, Al) or the leaching of polymer migrants can interact with the drug, leading to potency loss and new impurities, directly impacting shelf-life and the safety profile. This underscores the need for a Silica filler for chemical inertness in rubber, a core competency of specialized Rubber Functional filler Suppliers.
- Limitations of Traditional Solutions: Why Conventional Fillers Fail to Solve the Root Problem
- Traditional fillers or reinforcing agents often struggle to balance reinforcement, inertness, and processability. Some offer insufficient reinforcement, failing to effectively suppress fragmentation; others may introduce impurities or are chemically reactive themselves, becoming an “internal threat” to drug stability. The industry urgently needs a comprehensive solution that simultaneously provides physical reinforcement, ultimate chemical inertness, and excellent processing stability—a demand that can only be met by a sophisticated Rubber Functional filler Factory with advanced R&D capabilities.
Part 2: Technological Breakthrough – The “Triple Protection” Mechanism and Data Verification of RS915/RS925
First Shield: Physical Reinforcement – Ending the Era of Fragmentation with Nano-Scale Reinforcement
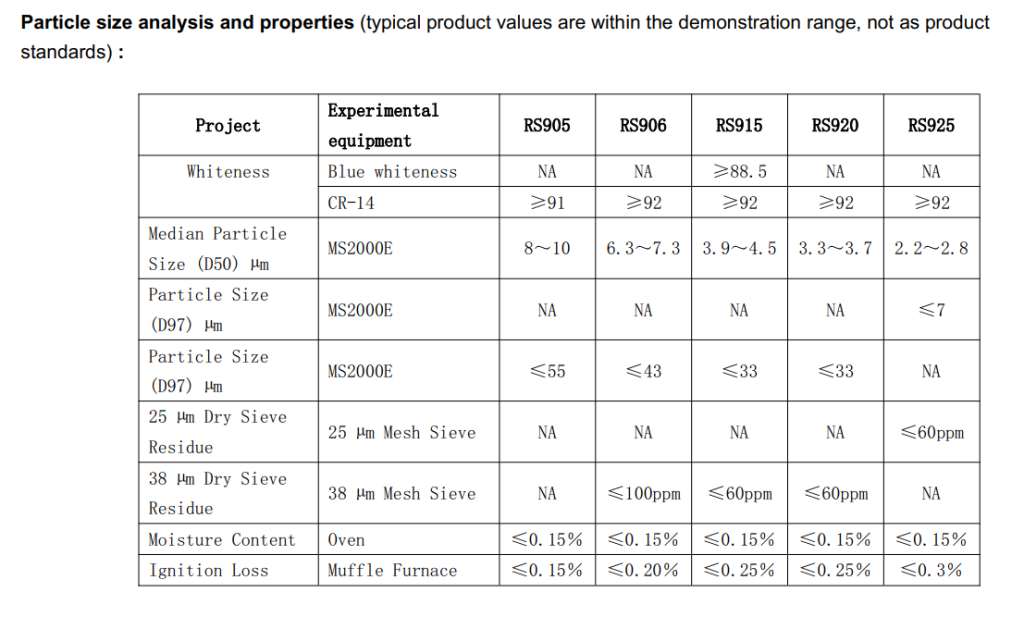
- Core Mechanism: The success of RS915 lies in its unique particle morphology and size distribution (D50: 3.9-4.5μm). This meticulously designed size allows it to form a dense and uniform three-dimensional nano-reinforcement network within the butyl rubber matrix, making it the ideal silica for medical butyl stoppers. This network effectively disperses and absorbs the stress generated during needle puncture, preventing stress concentration that leads to rubber tearing.
- Value Proposition:
- Superior Abrasion Resistance: The high loading of 60 phr RS915 in your provided formulation fully demonstrates its exceptional performance even at high fill levels. This translates to the stopper withstanding multiple punctures (including those required for Pre filled syringe stopper silica applications) with minimal generation of visible and sub-visible particles, significantly outperforming traditional formulations.
- Data Support: Its ≤60ppm residue on 38μm dry sieve directly confirms the virtual absence of large particles in the product that could cause initial contamination, ensuring cleanliness from the raw material end.
Second Shield: Chemical Inertness – Guarding Drug Molecule Activity with Ultimate Purity
- Core Mechanism: The foundation of chemical inertness is purity. RS915 boasts a SiO₂ content of ≥99.25% and critically low metal impurities like Fe₂O₃ ≤0.025% and Na₂O ≤0.02%. This extreme purity ensures it remains stable even in catalytic systems or complex drug environments, preventing it from becoming a catalyst for or participant in chemical reactions. It is, in essence, a Custom silica formula for medical applications where purity is non-negotiable.
- Value Proposition:
- Ensuring Drug Compatibility: The very low metal ion content minimizes the risk of catalyzing degradation in oxidation-sensitive drugs. This is crucial for vaccines, biologic proteins, and others.
- Excellent Insulation: The high purity also grants excellent electrical insulation properties, making it suitable for rubber components in specialized electronic medical devices.
Third Shield: Process Stability – Ensuring Zero Batch-to-Batch Variation with Superior Dispersibility
- Core Mechanism: RS915 exhibits excellent dispersibility, attributable to its particle surface properties and manufacturing process. During mixing and vulcanization, it disperses rapidly and uniformly within the rubber matrix, avoiding the formation of local weak spots due to filler agglomeration.
- Value Proposition:
- Consistency Assurance: Uniform dispersion ensures highly consistent physical (e.g., hardness, elasticity) and chemical properties across production batches, providing pharmaceutical companies with a stable supply chain and reducing quality fluctuations and risks during manufacturing. This reliability is what sets apart leading Rubber reinforcing filler Manufacturers China.
- High Yield: Good processability helps reduce production defects, directly improving production efficiency and yield, a key benefit for global Rubber reinforcing filler Suppliers China.
Part 3: Value Extension – From RS915 to RS925: Stepping into a More Refined Future
While RS915 provides the perfect solution for conventional and mid-to-high-end injectables, RS925 represents a more advanced technological direction.
- Finer Particles: RS925 has a D50 median particle size of only 2.2-2.8μm and a D97 ≤7μm. This enables even denser packing and a smoother product surface.
- Targeting Ultra-High-End Applications:
- Pre-filled Syringes: The most demanding field for fragmentation requirements, where RS925 can deliver a near-perfect puncture experience.
- High-end Ophthalmic Drugs, Cell Therapies: These high-value, micro-dose drugs demand the utmost in packaging compatibility, for which RS925’s ultra-high purity and fineness make it an ideal choice.
- Innovative Flame-Retardant Silicone Applications: Within your flame-retardant silicone systems, RS925’s high purity (≥99.2% SiO₂) and fine particle characteristics can not only maintain the inherent flame retardancy and insulation of the silicone but also significantly enhance its abrasion resistance and thermal conductivity, while providing the finished product with a superior surface finish. This makes it highly suitable for seals and insulating components in high-performance electronics, new energy vehicles, and other advanced fields.
III. Conclusion: Leading the Industry, Winning the Future Together
In an era of increasingly stringent pharmaceutical packaging regulations (e.g., evolving USP, EP requirements for leachables and particulates) and continuous innovation in drug development, every link in the supply chain determines the success of the final product and patient trust. Choosing a packaging material solution centered on GreenThinking® RS915/RS925 is not merely selecting a high-performance raw material; it is a commitment to “zero compromise” on drug quality, a strategy for proactive risk management facing the future, and the highest respect for patient safety.
Let us join hands, starting from the “first physical line of defense” for drugs, to collectively advance the reliability and safety of medical packaging materials to new heights, safeguarding global health.
Call to Action:
If you wish to delve deeper into the compatibility data of RS915 with your specific drug formulations, or to evaluate the potential of RS925 in your next-generation innovative rubber products, our technical expert team is ready to provide you with Custom silica formula for medical applications and comprehensive technical support. Partner with a leader in high-performance silica solutions to future-proof your products.
